Business requirements
- Manage version controlled documents
- The documents must be available in different languages
- Each document has his own initial language version
- The translated documents must be in relationship with the initial document
- Should a document version be subject to change, it is imperative that all related documents are updated accordingly
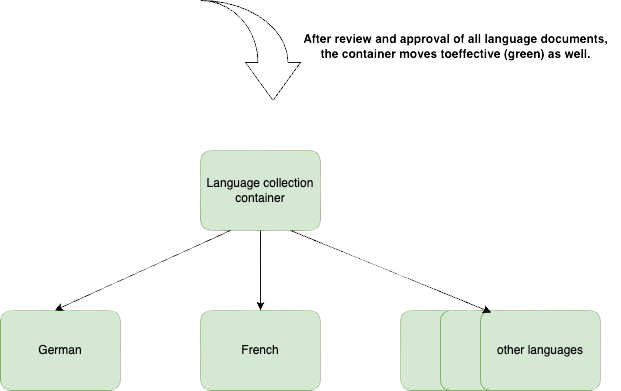
- After all documents version are updated and approved, the language collection is again effective
- Review and Approvals are manged and logged
Solution
A dedicated folder system is in place to manage all documentation and versions, ensuring compliance with requirements. The system incorporates a process for document approval and review, with tracking capabilities. The language of the documents is a key component of their metadata, with relevant information found on the metadata card and in the documents themselves.
The language collection container and document state are displayed on a timeline
The diagram below illustrates the various states of the owning connection container. Please note that a change to the state of the language collection container is triggered automatically when a document is updated.
List of the possible states:
- All language documents are in the effective state = Language collection container is also effective
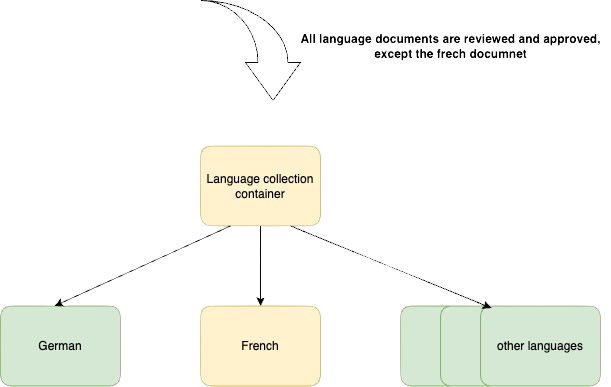
- One of the documents is in a review state = Language collection container is also under review
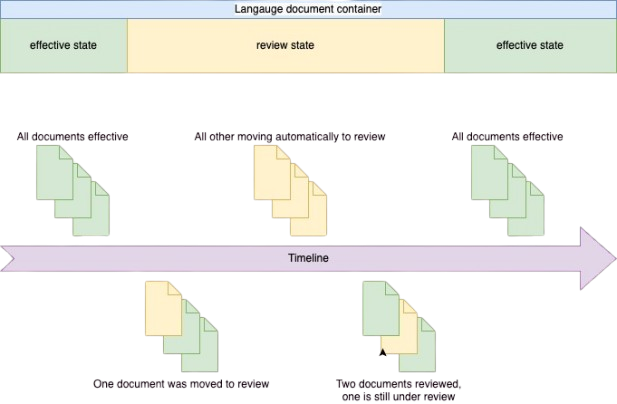
The graphics below provide a comprehensive overview of the document life cycle
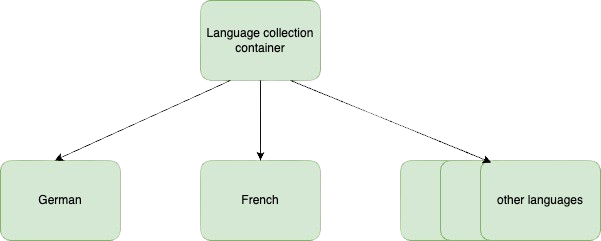
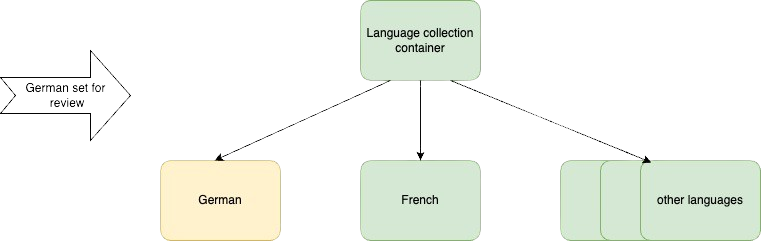
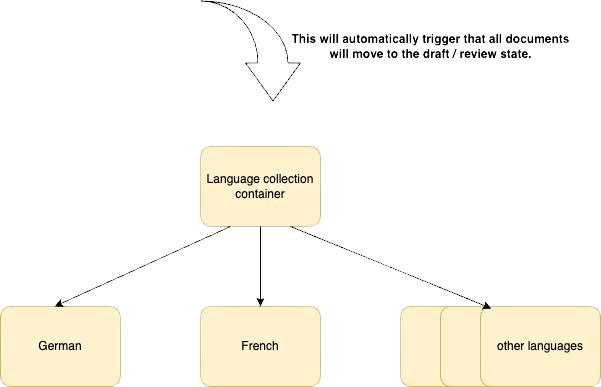
This diagram is great because it shows us that the language collection container and all the documents are in the ‘under review’ state.
Demo
Should you require further information on the version control of documents in M-Files, please refer to one of our recent webinars. They are available in German and French.
Conclusion
This solution is ideal for companies operating within a regulated and multilingual environment. It provides comprehensive tracking of document changes and ensures the integrity of the documents. Additionally, it offers extended logging capabilities to control document access.
The topics below can all be satisfied, especially with the use of the QMS template.
- Compliance to FDA 21 CFR part 11 or GDPR for example
- Qualified electronic signature
- Share documents with customer or partner with the use of M-Files Hubshare
- No Code Document Automation with M-File Ment
Should you require further information on integrations and interfaces, please do not hesitate to visit the M-Files website or contact us to arrange a demonstration and preliminary business analysis. We would be delighted to discuss how we can support you.
![Thumbnail [60x60]](https://www.dbi-services.com/blog/wp-content/uploads/2022/08/ATR_web-min-scaled.jpg)
![Thumbnail [90x90]](https://www.dbi-services.com/blog/wp-content/uploads/2022/08/GME_web-min-scaled.jpg)
![Thumbnail [90x90]](https://www.dbi-services.com/blog/wp-content/uploads/2022/08/PLE_web-min-scaled.jpg)